Stability Issues in Dietary Supplements
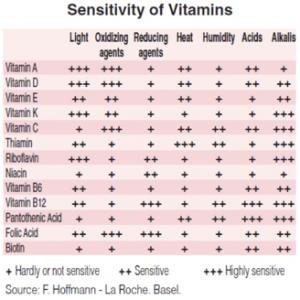
Most nutraceutical ingredients show degradation. Dietary supplements usually contain a mixture of ingredients, mostly natural products and plant extracts. Ingredients used to be of natural origin, but sometimes a synthetic product (identical to the natural one) is used due to some technical, cost or availability reasons.
In any case, all of them are products identified in nature, which means that they are not modified to obtain a soluble and stable compound readily absorbed by the body, once ingested. While this “optimization” is common in the pharmaceutical industry it is not in the nutraceutical sector.
Stability issues increase the cost and price of dietary supplements
As a consequence, many of the nutraceutical ingredients easily degrade, particularly when mixed with other ingredients and minerals. Although relevant work has been done to obtain formulations that avoid chemical degradation, in many cases the problem has not been satisfactorily solved (which is the case of most vitamins, CoQ10 and many other natural ingredients), giving place to safety and regulatory issues.
This is why optimised formulations increase hugely the price of the ingredient.
What are the regulatory issues associated with Degradation?
Regarding quality, dietary supplements must detail on the label their content in every relevant ingredient.
However, as many ingredients degrade, it is a common practice to introduce doses above what is stated on the label (20%, 50%, even 100% increase) to compensate for this degradation. As a result, when the final consumer purchases a supplement containing for instance 100 mg of an ingredient, the actual content can easily be between 50-200 mg.
As regulatory agencies are aware of this situation, they have limited the tolerances (deviation) of the real content from what is claimed on the label. EFSA (European Food Safety Authority) limits these deviations to +50%, -20%. In other words, if the label claims a content of 100 mg of a vitamin, the real content must be between 80 mg and 150 mg. On the other hand, FDA requires that naturally-occurring ingredients must be, at least in an 80% content of what is claimed on the label, and accepts a “reasonable” excess of vitamins.
The Confederation of Indian Industries published a guidance proposing overages for vitamins between 10% and 30%.
“Analysis by LabDoor of 75 of the top-selling multivitamins in the US has found that an average of 22% of products do not meet the claims made on their labels.”
“The latest Best Multivitamins Ranking from independent analysis service LabDoor also revealed that gummy/chewable multivitamins contain a 54% lower vitamin content and a 70% lower mineral content than standard multivitamins.”
(Nutraceutical Business Review, 8-May-2014)
What are the safety issues associated with Degradation?
For many ingredients, a Tolerable Upper Level of the ingredient for safe dose is established. Thus, when overdosing an ingredient on a dietary supplement to compensate for the product lost due to degradation, there are two potential concerns.
First, has the safety of the degradation products been evaluated?
Second, if a relevant overdose is used, and a customer is taking for instance 200 mg of an ingredient, instead of the 100 mg claimed on the label, the product safety should have been tested for this overdose. Moreover, can the excess of ingredients be accumulated in the body and lead eventually to toxicity effects under certain conditions?
“there are well-recognised harms from the ingredients of dietary supplements, especially when taken in high doses.”
(Australian Prescriber. An Independent review. 2 August 2021)
How can cocrystallization overcome the safety issue associated with stability of Dietary Supplements?
COCRYSTALS are a combination of components, an active ingredient and a coformer, which interact on a molecular level and crystallize in the same crystal lattice. This unique combination shows improved physicochemical properties compared with the free active ingredient crystal.

This process can be used to improve relevant properties, making the new cocrystal more stable (resistant to degradation) or more soluble in water than the parent compound.
Cocrystallization is a well-established process, not affecting the chemical structure of the ingredient, that has been explored in academia for many applications.
CIRCE Scientific is pioneering the use of this technology to improve nutraceutical ingredient properties with many relevant achievements in terms of enhanced stability and solubility.
Learn more about CIRCE Scientific achievements in improving stability of nutraceutical ingredients: Stability issues in vitamin D supplements; and Stability issues in CoQ10 supplements.





